Page 11 - reflections_dyslipidaemia_newsletter9_Final
P. 11
REFLECTIONS
Dyslipidaemia
Dyslipidaemia Global Newsletter #9 2025
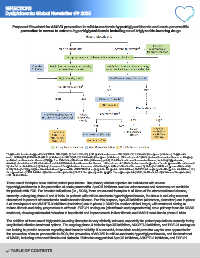
Proposed flowchart for ASCVD prevention in mild-to-moderate hypertriglyceridaemia and acute pancreatitis
prevention in severe to extreme hypertriglyceridaemia including novel triglyceride-lowering drugs Dyslipidaemia
a Triglyceride levels of approximately 200 to 500 mg/dL (2.3–5.6 mmol/L) (U.S. guidelines) or even 880 mg/dL (10.0 mmol/L) (European guidelines). Triglyceride
b
levels 500 mg/dL (5.6 mmol/L) (U.S. guidelines) to 880 mg/dL (10.0 mmol/L) (European guidelines). At least one of: 1) history of cardiovascular disease or imaging-
c
confirmed cardiovascular disease; 2) type 1 or 2 diabetes; 3) chronic kidney disease; and 4) 2 risk factors for cardiovascular disease, including hypertension,
smoking, elevated non-HDL cholesterol, and family history of cardiovascular disease. At least one of: 1) history of recurrent episodes of acute pancreatitis not caused
d
by alcohol or cholelithiasis; 2) history of recurrent hospitalisations for severe abdominal pain without other known cause; 3) history of childhood pancreatitis; and 4)
family history of hypertriglyceridaemia-induced pancreatitis. ANGPTL3/8-I, angiopoietin-like protein 3/8 inhibitors; ANGPTL4-I, angiopoietin-like protein 4 inhibitors; EU,
European Union; FCS, familial chylomicronemia syndrome; GLP-1-R-a, glucagon-like peptide 1 receptor agonists; MCS, multifactorial chylomicronemia syndrome;
U.S., United States.
These novel therapies have distinct patient populations. The primary clinical objective for individuals with severe
hypertriglyceridaemia is the prevention of acute pancreatitis. ApoCIII inhibitors such as volanesorsen and olezarsen are available
for patients with FCS. For broader indications (i.e., MCS), there are several therapies in all three of the aforementioned classes,
currently undergoing phase 2 and 3 trials. In patients with mild-to-moderate hypertriglyceridaemia, the focus is reducing remnant
cholesterol to prevent atherosclerotic cardiovascular disease. For this purpose, ApoCIII inhibitors (olezarsen, plozasiran) are in phase
3 of development and ANGPTL3 inhibitors (zodasiran) are in phase 2. MASH is another clinical target, with treatment aiming to
reduce fibrosis and delay progression to cirrhosis. FGF-21 analogues (efruxifermin and pegozafermin) are a primary focus for MASH
treatment, showing substantial reduction in hepatic fat and improvements in liver fibrosis and MASH resolution in phase 2 trials.
The addition of these novel triglyceride-lowering therapies is very clinically relevant, especially for patient populations currently facing
very limited effective treatment options. The ongoing phase 3 trials for ApoCIII inhibitors, ANGPTL3 inhibitors, and FGF-21 analogues
are looking to provide answers regarding their broader viability. If successful, these trials could pave the way for new approvals for
the prevention of acute pancreatitis in MCS, the prevention of ASCVD in mild-to-moderate hypertriglyceridaemia, and the treatment
of MASH, including advanced fibrosis and cirrhosis. Estimates suggest that ApoCIII inhibitors, ANGPTL3 inhibitors, and FGF-21
TABLE OF CONTENTS